본문
| Department | Biomedical Sciences |
|---|---|
| Major Field of Research | The central nervous system (CNS) consists of neurons and glial cells. Knowledge about fate specification of cells in the CNS is essential to develop therapeutic modalities for CNS diseases, especially neurodegenerative diseases. Whereas fate specification of neuron has been studied extensively, that of glial cells remains unclear. Our research group investigates the fate specification of glial cells, especially ependymal cells, in the zebrafish model using genetic and cell biological approaches. |
| zebrafish@chonnam.ac.kr | |
| Homepage | zfish.jnu.ac.kr |
Research interests
Seok-Yong Choi’s lab is performing research on the topics listed below using zebrafish as a model organism. Taking advantage of its easiness in carrying out drug screen, we are developing drugs related to respective research topic collaborating with Professor Jin Hee Ahn in Department of Chemistry at Gwangju Institute of Science and Technology (GIST).
1. Molecular mechanism of regeneration of motile cilia
- Motile cilia exist in ependymal cells in brain ventricles and spinal central canal, oviduct epithelial cells and respiratory epithelial cells, and are involved in flow of cerebrospinal fluid, migration of oocytes and removal of mucus, respectively. As such, defect in motile cilia leads to hydrocephalus, infertility and respiratory infection.
- We are investigate molecular mechanism underlying restoration of defective motile cilia and thus developing small molecules that regenerate motile cilia.
2. Development of Aryl hydrocarbon receptor (AhR) antagonists
- AhR was previously known as receptor of xenobiotics such as dioxin, yet its research is very active worldwide because it has been reported to be implicated in stem cell biology, immunity and infection.
- Using transgenic AhR reporter zebrafish and small molecule library, we have identified AhR antagonists. We are currently improving their efficacy by optimizing lead compounds.
- We recently found that AhR antagonist we developed exhibited anti-viral activity against SARS-CoV-2, virus causing COVID-19.
3. Molecular diagnosis of human genetic diseases
- Advent of Next Generation Sequencing (NGS) dramatically reduced time and expense required for sequencing of human genome, which makes it much easier to identify causative gene of human genetic diseases. However, functional study including research on animal model is critical to prove the causality.
- We are identifying causative genes responsible for human neurological genetic diseases and determining how mutations of these genes elicit the diseases, collaborating with Professors Myeong-Kyu Kim and Tai-Seung Nam in Department of Neurology at Chonnam National University Medical School.
4. Molecular mechanism of sex determination in zebrafish
- Sex is determined by either sex chromosomes or environmental factors such as tempature and density. However, neither sex chromosomes nor environmental factors have been known to be involved in sex determination to date.
- We recently found that lack of mitoPLD, a key player in mitochondrial fusion and piRNA generation, leads to gonadless zebrafish with male appearance, and are currently studying how mitoPLD regulates SD in zebrafish.
Publication
- Kim JT, Won SY, Kang K, Kim SH, Park MS, Choi KH, Nam TS, Denis SW, Ferdinandusse S, Lee JE*, Choi SY*, Kim MK*. ACOX3 Dysfunction as a Potential Cause of Recurrent Spontaneous Vasospasm of Internal Carotid Artery. Transl Stroke Res. 2020 Oct;11(5):1041-1051. * co-corresponding authors
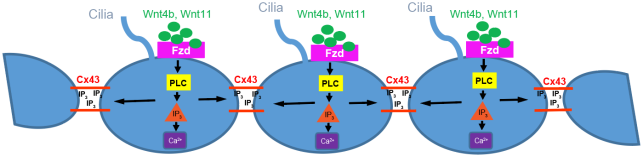
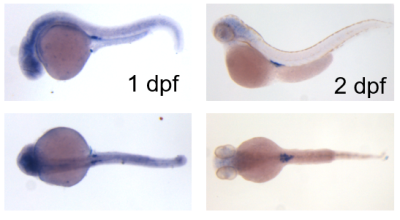
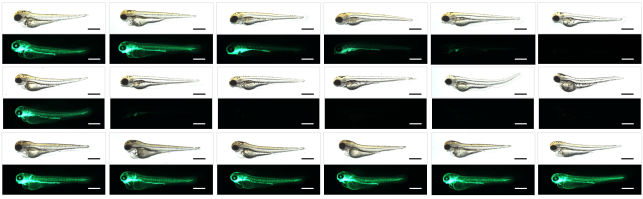
- Zhang J, Chandrasekaran G, Li W, Kim DY, Jeong IY, Lee SH, Liang T, Bae JY, Choi I, Kang H, Maeng JS, Kim MK, Lee T, Park SW, Kim MJ, Kim HS, Ro H, Bae YC, Park HC, Choi EY*, Choi SY*. Wnt-PLC-IP3-Connexin-Ca2+ axis maintains ependymal motile cilia in zebrafish spinal cord. Nat Commun. 2020 Apr 20;11(1):1860. * co-corresponding authors
- Nam TS, Zhang J, Chandrasekaran G, Jeong IY, Li W, Lee SH, Kang KW, Maeng JS, Kang H, Shin HY, Park HC, Kim S*, Choi SY*, Kim MK*. A zebrafish model of nondystrophic myotonia with sodium channelopathy. Neurosci Lett. 2020 Jan 1;714:134579.
- * co-corresponding authors
- Kang KW, Kim W, Cho YW, Lee SK, Jung KY, Shin W, Kim DW, Kim WJ, Lee HW, Kim W, Kim K, Lee SH, Choi SY*, Kim MK*. Genetic characteristics of non-familial epilepsy. PeerJ. 2019 Dec 19;7:e8278.
- * co-corresponding authors
- Jeong J, Kim KH, Kim DY, Chandrasekaran G, Kim M, Pagire SH, Dighe M, Choi EY, Bak SM, Kim EY, Shin MG*, Choi SY*, Ahn JH*. Identification of new aryl hydrocarbon receptor (AhR) antagonists using a zebrafish model. Bioorg Med Chem. 2019 Oct 1;27(19):115014.
- * co-corresponding authors
- Lee SH, Nam TS, Kim KH, Kim JH, Yoon W, Heo SH, Kim MJ, Shin BA, Perng MD, Choy HE, Jo J*, Kim MK*, Choi SY*. Aggregation-prone GFAP mutation in Alexander disease validated using a zebrafish model. BMC Neurol. 2017 Sep 7;17(1):175.
- * co-corresponding authors
- Lee SH, Nam TS, Li W, Kim JH, Yoon W, Choi YD, Kim KH, Cai H, Kim MJ, Kim C, Choy HE, Kim N, Chay KO*, Kim MK*, Choi SY*. Functional validation of novel MKS3/TMEM67 mutations in COACH syndrome. Sci Rep. 2017 Aug 31;7(1):10222.
- * co-corresponding authors
- Nam TS, Li W, Yoon S, Eom GH, Kim MK*, Jung ST*, Choi SY*. Novel NTRK1 mutations associated with congenital insensitivity to pain with anhidrosis verified by functional studies. J Peripher Nerv Syst. 2017 Jun;22(2):92-99.
- * co-corresponding authors
- Nam TS, Li W, Heo SH, Lee KH, Cho A, Shin JH, Kim YO, Chae JH, Kim DS, Kim MK*, Choi SY*. (2015) A novel mutation in DNAJB6, p.(Phe91Leu), in childhood-onset LGMD1D with a severe phenotype. Neuromuscul Disord. 25(11):843-51.
- * co-corresponding authors
- Nam TS, Kim JH, Chang CH, Yoon W, Jung YS, Kang SY, Shin BA, Perng MD*, Choi SY* and Kim MK*. (2015) Identification of a novel nonsense mutation in the rod domain of GFAP that is associated with Alexander disease. Eur J Hum Genet. 23(1):72-8.
- * co-corresponding authors